

Cancer: Heredity and genetic factors

Many cancers are part of a family history, within families that have been touched by the disease on several occasions.
In most cases, it is the result of chance or a combination of common factors (environment, similar lifestyle…)
But, for 5 to 10% of them, cancers are linked to a genetic predisposition, that is to say 20,000 new cases each year. The most frequent cancers are breast, ovarian and digestive cancers.
If you have cancer, or some of your family members have been affected, you may be wondering about genetic and hereditary factors.
What are the risks of developing cancer? What are the risks of passing it on to your children? In which cases and how to consult a specialist in oncogenetics?
I hope through this article to bring you some answers.
Genetic mutation
Genetic predisposition
Genes tell each of our cells how to function and how to repair themselves. They are located on the chromosomes.
Each chromosome is made up of a double helix of DNA organized according to a very precise sequence, the “genetic code“.
If the DNA is altered or there is an abnormality in this genetic sequence, this can lead to a genetic mutation and gene dysfunction.
Most of our cells are constantly dividing to replace those that die. This is how they reproduce. This process is “monitored” by specialized genes: “DNA repair genes” and “tumor suppressor genes”. They are essential to the functioning of the human body and, among other things, prevent the division of abnormal cells and repair damage to the cells’ DNA.
When these genes malfunction, they are no longer able to control the division of abnormal cells, which then multiply and become the starting point for cancer.
When a gene is defective and has a mutation, there are fewer “steps” for cancer to develop and therefore a greater chance of developing it than other people.
It is not a genetic alteration that occurs during one’ s life, the mutation is present from birth in all the cells of the body and can be transmitted from one generation to the next.
People in the family who do not have the genetic mutation are not at increased risk of developing cancer.
How is the genetic mutation transmitted?
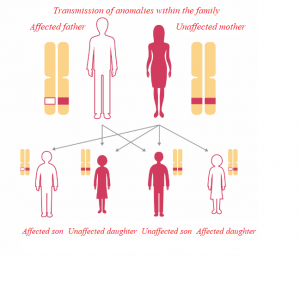 Every cell in our body contains 23 chromosomes in duplicate. For each pair of chromosomes, one copy comes from our mother, the other from our father. The genes, located on the chromosomes, therefore exist in duplicate in each of our cells, one coming from our mother, the other from our father.
Every cell in our body contains 23 chromosomes in duplicate. For each pair of chromosomes, one copy comes from our mother, the other from our father. The genes, located on the chromosomes, therefore exist in duplicate in each of our cells, one coming from our mother, the other from our father.
It is at the moment of fertilization that the chromosomes from the mother and the father come together to form the 23 pairs of chromosomes that will then be reproduced identically in each cell of our organism.
This transmission is said to be “autosomal dominant” (the gene is expressed as soon as it is present in the pair of chromosomes, even if the second copy does not carry any mutation).
The man or woman carrying a mutation is therefore also a carrier of the unaltered gene on the 2nd chromosome. Fertilization can therefore take place either with a gamete carrying the normal gene (with a 50% probability) or with a mutated gene (with the same 50% probability). The distribution is completely random at the time of the formation of the gametes. The probability of transmission of the mutation to each child is therefore 50%. The risk of transmission to each child is the same, whether you are a man or a woman.
If it is the normal gene that has been passed on, the child has not received the gene carrying the mutation, so he or she has no greater risk of developing cancer and will not pass on the mutation to his or her own children.
BRCAs and others
There is a wide range of genes identified in oncogenetics. The two genes whose mutations predispose to female cancers are BRCA1 and BRCA2 (Breast Cancer), discovered in 1994 and 1995 respectively. BRCA1 is located on chromosome 17 and BRCA2 on chromosome 13.
When functioning normally, BRCA1 and BRCA2 are repair genes. Their role is to repair or suppress a cell that is malfunctioning or multiplying in an uncontrolled manner. Deleterious mutations in BRCA1 or BRCA2 render them ineffective in these roles, predisposing the carrier to developing breast and ovarian cancers.
Men with a BRCA mutation also have an increased risk of breast cancer and, especially, prostate cancer.
Other genetic mutations predispose to breast and ovarian cancer.
For example, when they concern the genes BRIP1, FGFR2, TNRC9, MAP3K1, LSP1, CHEK2, PALB2, HCP or RECQL. The assessed risk is lower than for BRCA mutations, but these abnormalities are more prevalent in the population.
Screening
Assessing risk with the Geneticancer test
Geneticancer is an association dedicated to the fight against genetic and / or hereditary cancers. Laetitia Mendes (link to her book), carrier of a BRCA2 mutation, decided to found GENETICANCER when she realized that nothing answered the need for sharing experience of the “mutants”.
These people who, like them, are genetically predisposed to cancer, and who one day may be confronted with preventive surgery.
Geneticancer’s objective is to support, accompany, educate, inform, communicate and participate in the financing of research on genetic and/or hereditary cancers.
The association has set up a quick and simple questionnaire to help you discuss your risk of developing breast or ovarian cancer with your doctor.
This assessment tool is intended to be indicative only. It does not replace the advice of a specialist. Moreover, in certain circumstances, the use of this tool is not recommended. So make sure before you start that this questionnaire is adapted to your situation.
Oncogenetic consultation
-Who is it for?
An oncogenetic consultation can be proposed to a person suffering from cancer as well as to his or her family, depending on the type of cancer and his or her personal and family history. (link to e cancer website)
The oncogenetic consultation is primarily intended for the person who has developed cancer and presents clinical signs suggestive of a genetic predisposition: numerous cases of cancer in the family, development of cancer at an early age or several cancers successively or simultaneously. This person is called an “index case”.
Secondly, the consultation may be offered to a person who has been informed of a genetic alteration predisposing to cancer in a family member and who wishes to discuss this with the oncogenetics team. The test then offered to these relatives consists of determining whether or not they are carriers of the same cancer predisposition factor. This is called targeted testing.
It is often difficult to be diagnosed with a deleterious genetic mutation. If you feel the need, you can be accompanied by a psychologist or a psychiatrist who is used to working in oncology.
-How is it done?
The diagnosis of genetic predispositions to certain forms of cancer is carried out within the framework of the national oncogenetic system, which includes consultation sites and laboratories.
Currently in France, there are 125 oncogenetic consultation sites in 90 cities. They are accessible to people who have declared cancer as well as to their relatives. 25 laboratories are authorized to perform the analyses prescribed in oncogenetics.
Directory of consultations: https://geneticancer.org/wp-content/uploads/2018/11/annuaire_consultation_oncogenetique2018.pdf
Two modalities exist to search for a genetic alteration:
-either from blood or mouth cells, usually taken from a blood sample or a mouth swab
-or from a fragment of the tumor.
The samples taken are then sent to a specialized laboratory, which extracts the DNA and then “sequences” it, i.e. decodes the genes and the information they contain.
The data produced is analyzed by high-performance equipment, combined with the expertise of genetic biologists and clinical geneticists.
These genetic analyses are time consuming and can take several months.
An adapted medical follow-up
Individuals with a mutation benefit from personalized, multidisciplinary follow-up throughout their lives.
Oncogenetic follow-up programs were set up by the National Cancer Institute in part in 2009 (pilot experiments) and then in 2012 (national deployment).
They must conduct four main missions:
-Set up an individualized follow-up of people with a hereditary predisposition to cancer
-Coordinate this follow-up on a regional or even interregional level;
-Ensure access to multidisciplinary skills, either internally, within the establishments associated with the program, or externally, or in an alternating internal/external manner, according to the wishes of the individual or the logic of regional organization
-Ensure an activity of recourse and expertise for difficult cases.
Preventing risks
The close medical follow-up (breast follow-up and imaging) proposed in case of discovery of a deleterious genetic mutation is demanding. It does, however, make it possible to detect cancers at a very early stage, which makes the prognosis more favorable. However, follow-up does not reduce the risk, it only aims at detecting the disease as early as possible.
In certain circumstances, risk reduction surgery can be proposed.
Risk reduction surgeries
 -preventive mastectomy
-preventive mastectomy
Depending on your risk, calculated in a personalized way by your oncogeneticist, a double mastectomy may be proposed as a preventive measure. Prophylactic mastectomy or “preventive breast surgery” consists of the removal of the breast gland when you have not been diagnosed with cancer. This surgical decision is accompanied by a multidisciplinary follow-up including psychological follow-up and a reflection period.
In 95% of cases, the nipple and areola are preserved (unlike a mastectomy on a diseased breast).
The risk of breast cancer is then close to that of the general population.
-ovarian surgery
Ovarian cancer is very difficult to detect. This is why, in the case of a deleterious BRCA1 or BRCA2 mutation, preventive surgery is recommended.
As with prophylactic mastectomy, this option is offered based on your personal risk, family history, desires and concerns. Prophylactic ovarian surgery renders the woman permanently infertile.
There are several methods:
Bilateral salpingo-oophorectomy or adnexectomy, which involves removing the tubes and ovaries: this is the most commonly used and currently recommended method.
Salpingotomy, which consists in removing only the tubes and their pavilions (where the ovaries are born). This method has been studied but not yet tested in France.
Chemoprevention
Medical research is looking at drug-based, rather than surgical, prevention techniques. Studies have focused on Tamoxifen, which is commonly used in hormone therapy for breast cancer. Others are underway. And projects are bound to emerge as knowledge of genetic cancers progresses.
TO CONCLUDE
Many women have chosen to undergo risk reduction surgery in order not to live with a sword of Damocles hanging over their heads. A difficult and often painful choice that transforms the body in a heavy and irreversible way.
I sincerely hope that medical advances will one day allow women with a deleterious mutation to live serenely without having to resort to this mutilating surgery.
For more information, support and sharing around the issue of oncogenetics, I invite you to contact the association Geneticancer, to which we are pleased to have given our support.
“To accompany women with a genetic mutation and provide them with a gentle bubble in their surgery journey, we wanted to call on committed partners to offer them a “gene-free box” with adapted products.” Chistine – Geneticancer Auvergne Rhône Alpes Ambassador
To find an ambassador near you, click on this link:: https://geneticancer.org/nos-ambassadrices/

